An Intro to Lab-Grown Diamonds
We all have seen and know diamonds through out our life. Some of us are captivated with diamonds because of the way they shine. It is a timeless marvel of nature, symbolizing an everlasting love and sophistication
Now, a new contender has come into the picture., and it is the lab-grown diamond. Some of you might know that lab-grown diamonds are considerably cheaper compared to its natural counterpart and start wondering whether it is a fake imitation (TLDR : it's not!). This article will talk in details about what is a diamond and what are the difference between those of lab-grown vs natural.
What is a Diamond?
Scientifically speaking, a diamond is " a solid form of pure carbon with its atoms arranged in a crystal ". There are 2 parts that we need to explain and let's break that down in a more simpler way.
It is carbon! Carbon is one of the elements from chemical periodic table (that we all know and love from our high school chemistry class). It is a very common element that we find day-to-day and it makes up the life forms we have on Earth. You, me , your dog, and every living organism that we interact on day-to-day basis are made out of carbon atoms.
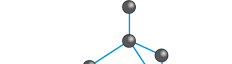
But if we are all made out of carbon, then why don't we display properties like diamond? (I don't shine like a diamond). Well, the answer is in the second part. Diamonds are crystal. Every carbon atoms in our body are bonded in a very specific way and so does diamond. To create diamond, we need 4 Carbon atoms, bonded into each other forming a tetrahedral shape (pyramid-like shape). And that 4 atoms will each bond to another 4 atoms and so on and so forth, forming a crystal structure that we call diamond.
(source : minichemistry.com)
So long story short, diamond is a group of 4 carbon atoms arranged in a very specific structure.

Natural vs Lab-Grown
Now that we've covered the basics, let's talk about the difference between natural vs lab-grown diamonds. The main difference is in the way those two are created. To create a diamond, millions of tons of pressure need to be applied. With natural diamond, the pressure is created by layers and layers of earth's mantle. Remains of living organisms, through millions of years of buried deep into earth's mantle, transformed into diamonds.
With the advent of modern technology, we now realize that we can simulate that high pressure in a laboratory. Through a process called Chemical Vapor Deposition (CVD) High Pressure-High Temperature (HPHT), that we'll discuss later, we can create the environment needed to create diamonds without having to wait millions of years or under the pressure of millions tons of rocks.


